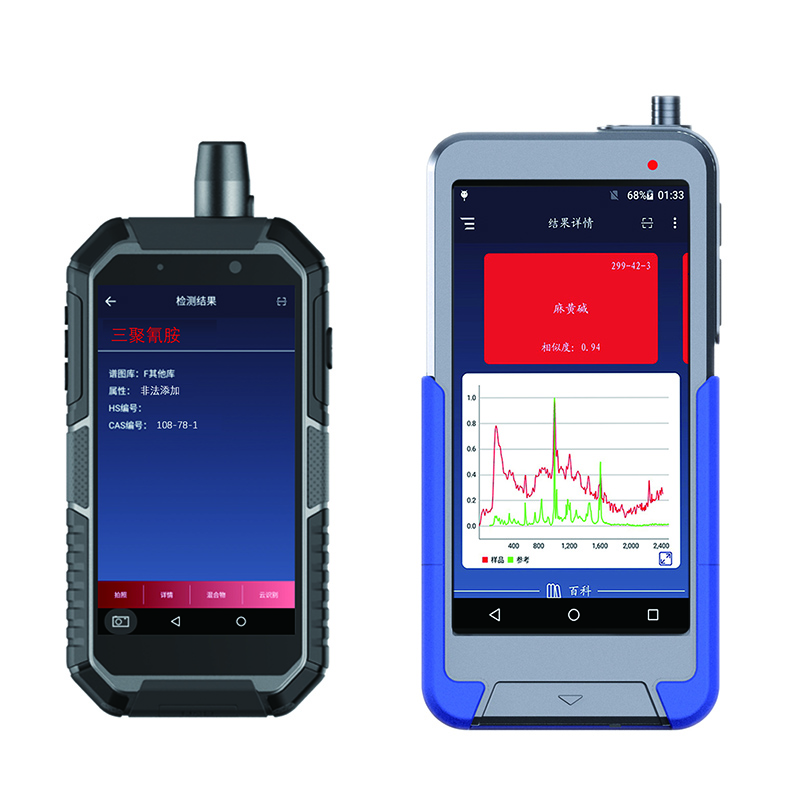
RS1000DI RS1500DI handheld Raman Identifier
★ A wide range of detection, chemical, biochemical raw materials, and pigments can be identified
★ It can be directly tested through glass, woven bags, paper bags, plastics, and another packaging (RS1500DI)
★ Small and lightweight, it can be flexibly moved in warehouses, material preparation rooms, production workshops and other sites
★ Quick response and identification can be completed in seconds
★ No need to take sampling, no need to transfer raw and auxiliary materials to the sampling room, which can avoid sampling contamination
★ Accurate identification, using advanced machine learning algorithm, strong specificity
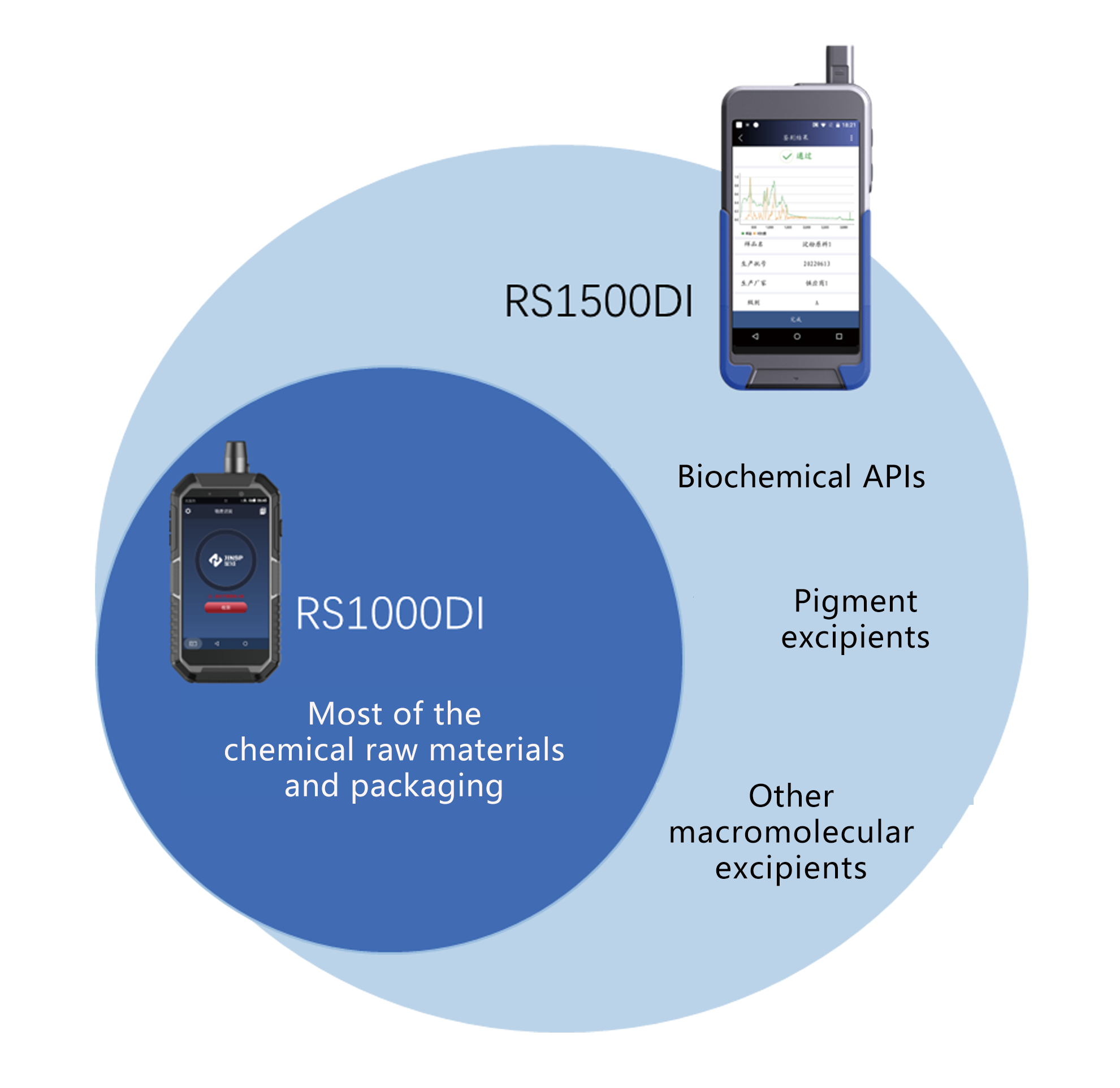
RS1000DI&RS1500DI
• Chemical raw materials: aspirin, acetaminophen, folic acid, niacinamide, etc.
• Pharmaceutical excipients: salts, alkalis, sugars, esters, alcohols, phenols, etc.
• Packaging material: polyethene, polypropylene, polycarbonate, ethylene-vinyl acetate copolymer
RS1500DI
• Biochemical APIs: amino acids and their derivatives, enzymes and coenzymes, proteins
• Pigment excipients: carmine, carotene, curcumin, chlorophyll, etc.
• Other macromolecular excipients: gelatin, microcrystalline cellulose, etc.
RS1500DI:
| Specification | Description |
| Technology | Raman Technology |
| Laser | 1064nm |
| Weight | 730g(including battery) |
| Connectivity | USB/ Wi-Fi/ 4G/ Bluetooth |
| Power | Rechargeable Li-ion battery |
| Data format | SPC/ txt/ JEPG/ PDF |
RS1000DI:
| Specification | Description |
| Laser | 785nm |
| Weight | <500g(including battery) |
| Connectivity | USB/ Wi-Fi/ 4G/ Bluetooth |
| Power | Rechargeable Li-ion battery |
| Data format | SPC/ txt/ JEPG/ PDF |
1. International Pharmaceutical Inspection Cooperation Program (PIC/S) and its GMP Guidelines:
Appendix 8 Sampling of Raw Materials and Packaging Materials The identification of the whole batch of materials can be confirmed only after the identification test is carried out on the samples in each packaging container.
2. US FDA's current good manufacturing practice US FDA GMP:
FDA 21 CFR Part 11: For each component of a drug, at least one identification test shall be carried out;
FDA Inspector's Instruction Manual: Conduct at least one specific identification test for each batch of each raw material.