1 Background
The development of polysilsesquioxanes (PSQs, general formula (RSiO1.5)n) is currently a hot ffeld in the materials chemistry community, thanks to their molecular-level organic–inorganic hybrid features. At present, PSQ materials have been widely used in catalysi, biological diagnosis and targeted therapy, adsorption/separation, optoelectronics, superhydrophobic materials, and insulating coating. The excellent versatility of PSQs arises from the tailorable functional groups (R = alkyl, amino, mercapto,etc.), good thermal and chemical stability, biocompatibility, and hydrophobicit. Almost all existing studies are performed in the acid conditions, whereas the study on the base-catalyzed hydrolysis kinetics is scarce. In addition, previous studies mainly focused on the determination of activation energy and pre-exponential factor, but lacked a systematic study on the reaction orders with respect to alkoxysilane, water, and catalyst.
2 Experimental protocol
In this paper, the hydrolysis reaction of MTMS was monitored in situ by a Raman spectrometer (RS2000, JINSP Co., China) with an excitation wavelength of 785 nm, a laser output power of 400 mW, and a resolution of 6 cm-1. The solubility of MTMS in H2O was measured by Raman spectroscopy, and a mathematical model of the heterogeneous hydrolysis process of MTMS droplets was created based on the hydrolysis kinetics and solubility data.
3 Results and discussion
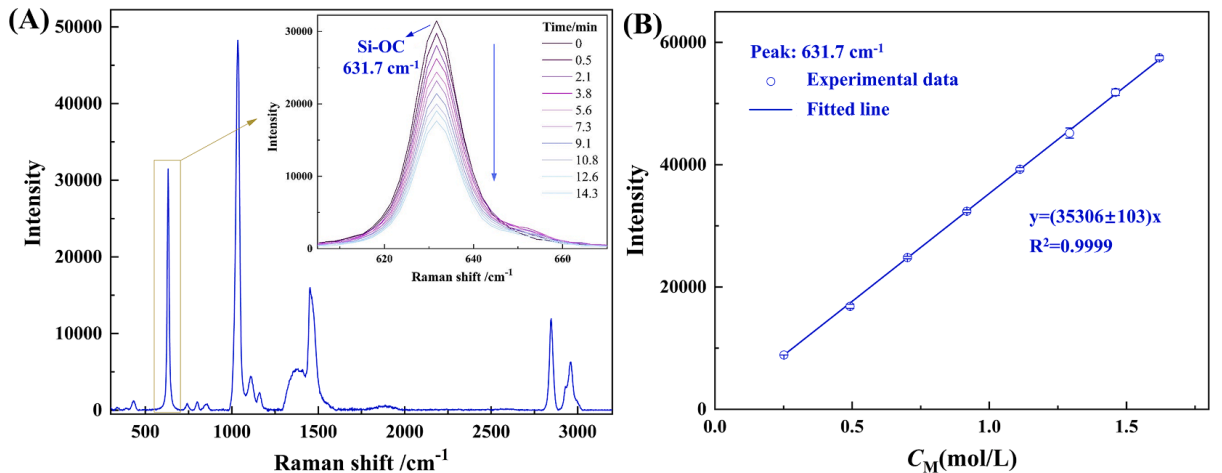
(1) Fig.1A shows a representative Raman spectrum of MTMS methanol solution. The peak at 631.7 cm-1 could be attributed to the Si-OC symmetric stretching vibration of the solvated MTMS, and the intensity of the characteristic peak gradually decreased with the reaction time, which indicated the degree of the hydrolysis reaction. This peak was used as the quantitative peak, and the calibration curve of MTMS methanol solution was shown in Fig.1B. It could be seen that Raman quantiffcation had little relative error (<1%) and the results were sufffciently reproducible. There was a good linear correlation between the characteristic peak intensity and MTMS concentration.
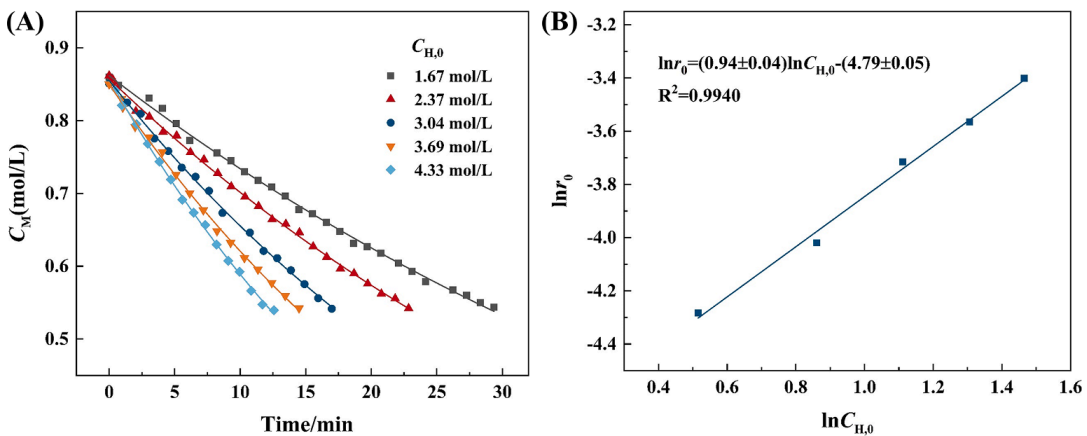
(2) In this paper, the kinetic parameters of the early polycondensation reaction were determined using the same method as the hydrolysis reaction. With a constant temperature and ffxed concentrations of MTMS and NH4OH, the initial concentration of H2O was changed. Using the above-mentioned measuring method and data processing method, the variation of MTMS concentration with time at different CH,0 and the plot of lnr0 as a function of lnCH,0 are shown in Fig.2 The reaction order with respect to H2O is 0.9.
Fig. 1. (A) Representative Raman spectrum of MTMS methanol solution (inset: variation of the characteristic peak intensity with the reaction time), (B) calibration curve of MTMS methanol solution.
Fig. 2. (A) Variation of MTMS concentration with the reaction time under different initial H2O concentrations (CN=0.33 mol/L, T: 15 ◦C), (B) plot of ln r0 as a function of lnCH,0.
4 Conclusions
(1) In this paper, the hydrolysis kinetics of methyl trimethoxysilane (MTMS) catalyzed by alkali was quantitatively studied by in-situ Raman spectroscopy.
(2) Raman spectroscopy, owing to the advantages of short measuring time, in-situ, real-time, nondestructive, high sensitivity, low reagent consumption, and low interference from water, demonstrates a great potential in the qualitative and quantitative researches. The RS2000 portable Raman device (JINSP Co., China) with integrated high-performance transmission spectrometer ST100S (JINSP Co., China) enables rapid and real-time monitoring of the reaction process, and seems to be a powerful tool for the kinetic study of the rapid MTMS hydrolysis reaction.
5 Sources
6 Product recommendations |ST100S Transmission spectrometer

(1) Product Introduction
The spectrometer uses a volume-phase holographic (VPH) grating with a diffraction efficiency of nearly 90%, combined with an optical design optimized to the diffraction limit, to provide excellent imaging results. At the same time, it is equipped with a research-grade deep-cooling area array CCD to ensure extremely high sensitivity and SNR, and supports multi-channel acquisition with a resolution of up to 3cm-1.
(2) Product Advantages
In-situ, pollution-free, fast and real-time online detection
Efficient diffraction, superior imaging, high-performance camera, high resolution
For more details, please visit the official website of JINSP Co. Ltd: Best ST100S Transmission Imaging Spectrometer manufacturers and suppliers | JINSP (jinsptech.com)
Article Link:
C. Han, T. Tang, J. Deng, G. Luo, Quantitative determination of base-catalyzed hydrolysis kinetics of methyltrimethoxysilane by in-situ Raman spectroscopy, Chemical Engineering Journal 446 (2022) 136899.
DOI:10.1016/j.cej.2022.136889
https://doi.org/10.1016/j.cej.2022.136889
Post time: Jun-06-2024